THE SCIENCE
The Science
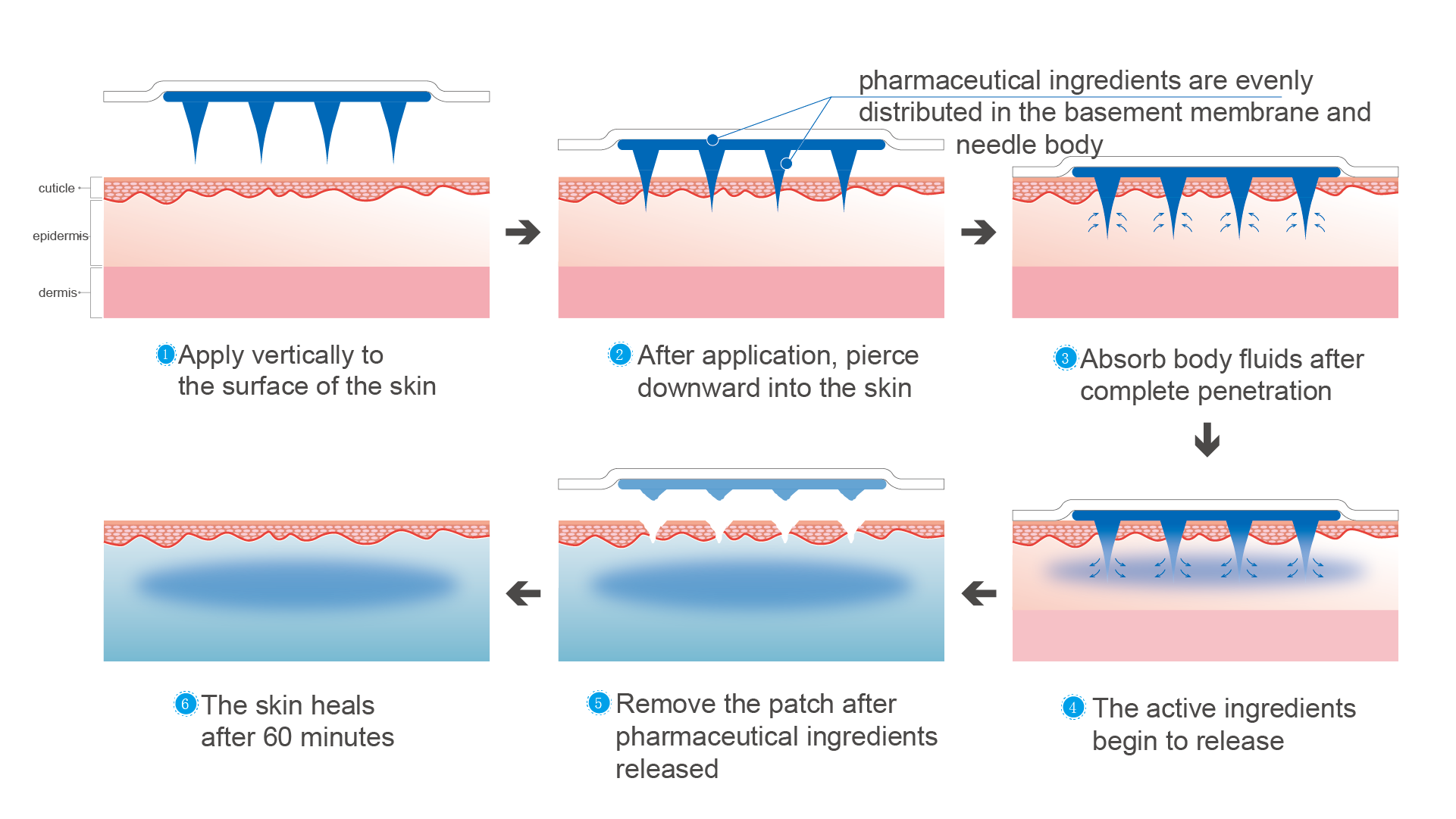
MN TDDS achieves drug delivery through the skin outside the body surface, which is the most convenient way of administration and also the most patient compliant way of administration. However, the industrialization of this method has been very challenging world widely for decades, which fully demonstrates the great difficulty of implementing this technology.
Here are some key challenges:
1���、Volume
Like all dosage forms, it is a combination of ingredients, but this time using molds to create needles instead of tablets, and they will be pricked into skin and dissolve there. Painless and less invasive are the original goals, so needles must be tiny which left a very limited space to contain APIs. At the meantime, size of patch cannot be increased due to their invasive principle. 1mg has been a boss for many challengers.
2���、Mechanical strength of needles
Mechanical strength has been a key factor to get through corneum and reach epidermal layer. The delivery will eventually fail if it cannot meet the requirement to complete such mission. The corneums of animals (including human) is significantly different. The mechanical strength of needles must be balanced to redeem such gap. It also need to be effective enough at anywhere of human body and still get the required human PK/PD results. This can be a key reason for poor consistency in clinical trials.
3���、Efficiency of MN TDDS
MN TDDS is a very good way to use drugs, but efficiency has always been a foundation of everything. Developers must understand the pattern of MN efficiency before rushing into later investigations. It will be more than enough at mice or rats but quickly reducing while the animal modules getting closer and closer to human. The worse thing is that bad efficiency normally requires more volume, which is insufficient. This will eventually lead project to its failure.
4���、Uniformity of results
Principle of MN TDDS is easy to understand but difficult to actualize. Uniformity is the soul of this platform and it has a bad track record. Differences from individuals will bring significant uncertainty to efficacy. This is a crucial reason of why we have not seen a single MN drug launched. June 2022, ZOSANO (NSDAQ: ZSAN) announced its bankruptcy, because its clinical asset is challenged by FDA, due to the differences between individuals. This is another big challenge for all researchers of MN TDDS. There is no reliable vendor of facilities for this platform yet globally. Designation of a uniformed and sterilized GMP facility, along with the quality system are the pre-requests to develop a MN candidate.
These are only four most important issues to be solved before clinical investigation, but absolutely not the difficulties which could be far more than these. It has been 66 years since this principle has been suggested by prof. Wagner in 1958. Humans are rarely patient like this. Some people believe that it can be theoretically only, but it is, in fact, developing quickly. There were 37 papers about “microneedle” in PubMed back in 2006, and this number increased to 490 in 2020.
MN TDDS is a very complex academic, technical, and engineering system. It involves multiple sciences such as pharmacy, chemistry, materials science, mechanical equipment, technology, engineering, etc. Dissolvable MN is a complex injection which is more complicate than LNP or PLGA, and probably the most challenging task ever because theory of LNP and PLGA are suggested later yet verified already. The key issue here is about the industrialization of this technology instead of some research in lab. It requires systematic knowledge, theory, and team in order to achieve.
BioQingLam values and respects the difficulty and characteristics of this technology. Here, we have deployed professional talents in formulation, process, equipment development, mold development, quality control, GMP compliance, and other areas. All the works strictly followed the system methodology guidance of 6 Sigma Design for Excellence DFSS, integrating GMP drug development and production compliance requirements into the entire technical process.
Here are some of the indicators we have already achieved:
Still Increasing Volume: up to 15mg of GLP-1+ kind of API in a single coin size patch;15mg for PD-L1
Increasing and narrowing range of bioavailability: semaglutide as an example, >90% in rat & ~61% in human
Large volume for SMEs to satisfy multiple strengths
Content uniformity: A+2.2S < 15, able to meet the toughest regulatory requirements
Regulatory Compliancy: Global only MN sterilized GMP plant today with all tailored facilities